Cruising the Energy Bands
New kind of atomic probe could be boon for quantum computing.
A team of Lincoln Laboratory and MIT campus researchers may have found a way to overcome a key barrier to the advent of super-fast quantum computers, which could be powerful tools for applications such as code breaking.
One approach involves superconducting devices that, when cooled to temperatures of nearly absolute zero (–273 degrees C), can be made to behave like artificial atoms—lithographically defined and fabricated micron-scale devices that behave like atoms by exhibiting specific, discrete energy levels. (Picture an elevator that can stop at the floors of a building but not in between.) But traditional scientific techniques for characterizing—and therefore better understanding—atoms and molecules do not necessarily translate easily to artificial atoms, says William Oliver of Lincoln Laboratory's Analog Device Technology Group and MIT's Research Laboratory for Electronics (RLE).
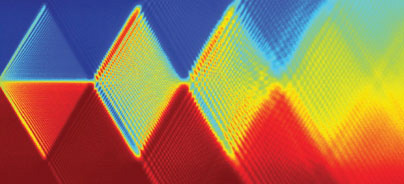 The colorful patterns formed by the response of superconducting "artificial atoms" to a new probe called amplitude spectroscopy serve as an identifying fingerprint for a given atom. The colorful patterns formed by the response of superconducting "artificial atoms" to a new probe called amplitude spectroscopy serve as an identifying fingerprint for a given atom.
|
In the September 4 issue of Nature, Oliver and colleagues reported on a complementary approach, called amplitude spectroscopy, that provides a way to characterize quantum entities over extraordinarily broad frequency ranges. This procedure is "particularly relevant for studying the properties of artificial atoms," Oliver says.
Characterizing energy levels is fundamental to the understanding and engineering of any quantum system. Ever since Isaac Newton showed that sunlight could be dispersed into a continuous color spectrum, this has been done through analysis of how an atom responds to different frequencies of light and other electromagnetic radiation—a technique known generally as spectroscopy. Frequency-dependent absorption and emission spectroscopy played a fundamental role in the development of quantum mechanics. But the artificial atoms have energy levels that correspond to a very wide swath of frequencies, ranging from tens to hundreds of gigahertz, making the method costly and difficult. "The application of frequency spectroscopy over a broad band is not universally straightforward," Oliver says.
Better knowledge of these superconducting structures could hasten the development of a quantum computer. Each artificial atom functions as a "qubit," or quantum bit. A qubit can be in multiple energy states at once: it would not be simply a one or a zero (like the electronic switches in a conventional computer) but rather in a sort of hazy combination of both states—a manifestly quantum effect. (It's akin to the famous paradox of Schroedinger's quantum cat, which is considered to be both alive and dead at the same time until an observation is made, simultaneously creating and revealing its true condition.) This odd behavior, inherent to the quantum nature of materials at the atomic level, is what gives quantum computing such promise as a paradigm-busting advance.
Amplitude spectroscopy provides a way to characterize quantum entities over extraordinarily broad frequency ranges. The approach gleans information about such a superconducting artificial atom by probing its response to amplitude rather than frequency. The driving source applies radiation at a fixed frequency, thereby circumventing many of the challenges associated with a broadband frequency-based approach. The applied radiation pushes the atom through energy levels; the larger the amplitude, the further through the energy levels the atom goes. "The technique utilizes Landau-Zener transitions at the points where energy levels cross," Oliver explains. An artificial atom can thus hop between energy levels at these crossing points, provided it is being driven quickly enough to jump the energy gap. Since the crossing speed is proportional to both amplitude and frequency, one can fix the frequency at a benignly small value, and then compensate it with a large amplitude. The larger the gap, the faster one must drive the atom to jump the gap and so the larger the amplitude required.
The Landau-Zener transitions also form Schroedinger cat states, a second key feature of amplitude spectroscopy. When the atom jumps an energy gap, it generally neither stays completely in the original level nor jumps completely to the other level. Instead, it forms a quantum superposition of the two levels, the weighting depending on the gap size and the crossing speed. The driving field pushes and pulls the state through a given crossing many times, and this state quantum mechanically interferes with itself. The resulting radiation emitted by the artificial atom therefore exhibits quantum interference patterns. These patterns, which Oliver calls "spectroscopy diamonds" because of their striking geometric regularity, serve as fingerprints of the artificial atom's energy spectrum.
Oliver's coauthors on the Nature paper are David Berns, a graduate student in physics and RLE; Mark Rudner, a graduate student in physics; Sergio Valenzuela, a research affiliate at MIT's Francis Bitter Magnet Laboratory; Karl Berggren, the Emanuel E. Landsman Career Development Associate Professor in the Department of Electrical Engineering and Computer Science (EECS); Leonid Levitov, professor of physics, and EECS professor Terry Orlando. |